A. UNDERSTANDING THE DEFINITION OF LIPIDS
(FATS)
Lipids (fats) is a not water-soluble chemical
compound composed by the elements carbon (C), hydrogen (H) and oxygen (O).
Lipid (fat) is hydrophobic (insoluble in water), to dissolve the lipid (fat) is
required special solvents such as ether, chloroform and benzene. like
carbohydrates and protein, lipid (fat) is also a source of energy for the human
body. Lipid (fat) also as basic component for our body tissues for contributing
to build cell membranes and membrane of some organelles. The weight of the
energy produced in lipid (fat) 2 ¼ times larger than carbohydrates and protein.
1 gram of lipid (fat) can produce 9 calories, while one gram of carbohydrate
and protein produces only 4 calories. During digestion, lipid (fat) is broken
down into fatty acids and glycerol to be absorbed by the digestive organs and
then taken to the organs that need it.
B. THE FUNCTIONS OF LIPID (FAT)
Lipid (fat) has many functions, some important
functions for the body are:
- As the energy reserves in the form of fat cells. When we consume excessive lipid (fat), then the lipid (fat) will be stored in various places for example in the lower layers of skin to be used as energy reserves.
- Important protective organ during a shock because it has a structure like a pillow.
- It protects the body from environmental temperature changes. Lipid (fat) could protect against low temperatures.
- One of the basic materials needed for the production of vitamin, hormones, cell membranes and the membranes of cell organelles.
- Solvents of the A, D, E, and K vitamin.
- Optimize the digestive function, fat can slow down the digestion process so that hunger does not appear too quickly.
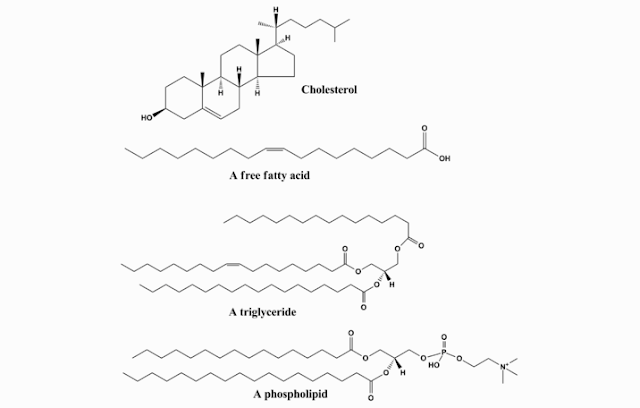
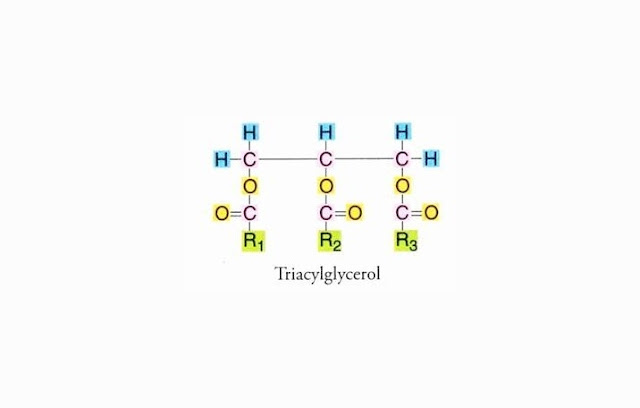
C. CHEMICAL STRUCTURE OF LIPID (FAT)
Components that form lipid (fat) are Carbon (C),
hydrogen (H) and oxygen (O) element. Lipid (fat) consists of three fatty acids
and one glycerol. Lipid (fat) Chemical Structure is:
 |
| CHEMICAL STRUCTURE OF LIPIDS (FATS) |
When all the three R1, R2 and R3 structures are the
same, it is called a simple lipid (fat), but if different then called lipid
(fat) mixture.
D. THE CHARACTERISTICS OF LIPID (FAT)
1. Lipid (fat)
Physics Properties
saturated fat has higher melting point than the
unsaturated fat. Lipid (fat) melting point depends on the length of their
carbon chain. For example, beef fat melts at a temperature of 49 degrees
Celsius and return to solid at 36 degrees.
Neutral lipid (fat) is not soluble in water, but
dissolves well in chloroform and benzene. Hot Alcohol is also a good solvents
for lipid (fat), but lipid (fat) is not very soluble in cold alcohol.
2. Lipid (fat)
Chemical Properties
a. Saponification
Reaction
Lipid (fat) can be hydrolyzed in various ways. One of
those ways is with alkali. Well The process of Lipid (fat) hydrolysis using
alkali is called saponification. The result of the hydrolysis is salt fatty
acids, also known as something we called “soap”.
b. Halogenated
reaction (iodine)
Unsaturated fatty acids, either its free or bound as
esters in the lipid (fat) around you adduct the halogen to the double bonds.
Because the temperature of lipid (fat) absorption is proportional to the number
of double bonds in the fatty acid, so the amount of halogen can be determine by
the degree of unsaturation. The degree of unsaturation is measured by its
iodine number,by how much iodine can react with 100 gr of lipids (fats).
Therefore, the more double bond are, the greater its iodine number.
c.
Hydrogenation reaction
The process of converting oil into lipid (fat) known
as hydrogenation (hardening process), it is done by bubbling pressure hydrogen
(1,75kg / cm2) into the hot oil (200 degrees Celsius) containing dispersed catalyst
nickel.
E. TYPES OF LIPID (FAT)
1. Based on
Sources of Fat
Divided into two :
- Animal fats, which is fat from animal products.
- Vegetable fats, are fats that are derived from plants.
2. Based On
The Chemical Structure
- Simple fat, fat that is composed by triglycerides, ie three fatty acids and one glycerol. Examples of these fats are waxes and oils.
- compound fat, which is fat that consists fatty acids and additional groups other than fat. Examples are lipoproteins (protein) and phospholipid (containing phosphate).
- Derivatives fat, fat is a component from the hydrolysis of lipids. Examples are cholesterol and fatty acids.
3. Based on
the chemical bond
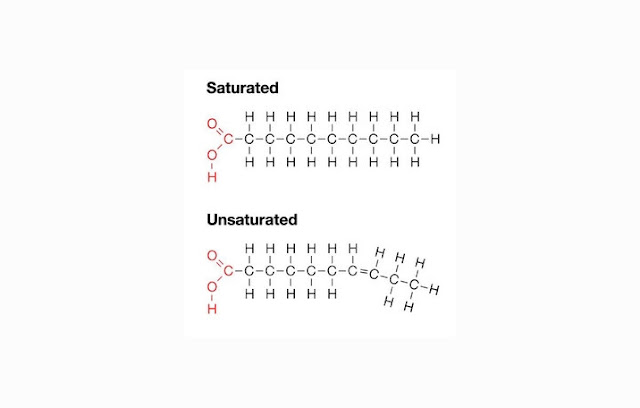
- Saturated fat, the fat structure with a single bond hydrocarbons that are harmful to the human body because it can be attached and clot which can impair blood circulation system. Saturated fat is from animal product, such as meat, milk, etc.
- Unsaturated fat, which is fat with one or more bonds (double) hydrocarbon structure that can benefit the body. Unsaturated fats are mostly derived from plants, for example fat from avocados and nuts.
 |
| SATURATED AND UNSATURATED LIPIDS (FATS) |
F. METABOLISM AND DIGESTION PROCESS OF
LIPIDS (FATS)
Fat digestion process is longer than carbohydrates
and protein. This is caused by the arrangement of long chain fatty molecules
and its strong ties. At the moment food enters the mouth, the teeth do its job
to crush and grind the fat mechanically. On the underside of the tongue, there
are the glands that produce lipase enzymes, these enzyme breaks down fat in the
mouth into a simpler form. After that, the swallowing process will carry fat
through the esophagus, then headed to the stomach. In the esophagus and stomach,
fat is not digested because there are no enzymes that can digest them, so the
fat simply mixed with other foods and stored temporarily in the stomach.
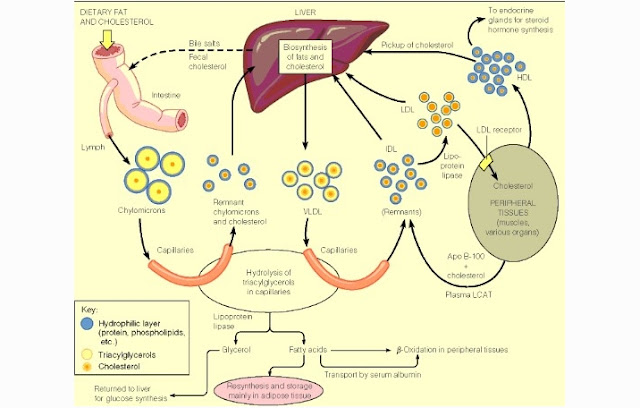
Fatty acids is being absorbed by the small intestine
mucosal cells by diffusion. In the cell, fatty acids and glycerol are resintesis
(recombination) into triglycerides. Cholesterol is also experiencing esterification
into cholesterol esters. Triglycerides and cholesterol ester are surrounded by
a protein then unite into chylomicrons.
Protein called apoprotein are the constituent of chylomicrons coat. Protein
coat prevents the union between the fat molecules and form a large circle which
can interfere to the blood circulation.
After Chylomicrons get out from the of the intestinal
mucous cells, they are transported through the lymphatic system into the blood
circulation. Chylomicrons levels increased 2-4 hours after eating. Chylomicrons
in the blood is hydrolyzed by the endothelial lipase enzyme into fatty acids
and glycerol. fatty acids that are released from chylomicrons stored in fat
tissue or peripheral tissues. Chylomicrons which has lost free fatty acids
contain much cholesterol and remain in the circulation called a remnant chylomicron
and eventually this remnant chylomicron transport to the liver and degraded in
the lysosome. While glycerol is absorbed directly into the portal hepatica
vein.
Free Fatty acids is used as a source of energy or
stored in the form of neutral fats or triglycerides. Hepar utilize fatty acids
as an energy reserve in the form of cholesterol, triglycerides or stores as fat
tissue or can also be converted into protein or amino acids.
Out Of the total fat that we consumed, 95% will be absorbed by the body and the other
5% will go to the large intestine and excreted through the anus.